An acidic oxide sulfur dioxide combines with water vapor in the air to produce sulfurous acid in the following reaction. This is not very soluble so rocks dont dissolve very quickly.

A Substitute Formulae For Names And Balance The Following Equation Calcium Carbonate Reacts Youtube
Calculate the average rate of reaction in a g s-1 b mol s-1 c cm 3 s-1.
. Testing for Presence of a Sulfate ion BaCl2 solution acidified with hydrochloric acid is used as a reagent to test for sulphate ions. The marble reacts to give. This neutralization reaction will result in the formation.
HNO 3 nitric acid. 2HCl CaO CaCl 2 H 2 O. HCl is a very strong acid that is corrosive and hazardous.
If we look at the formulas of different acids we can see that they all contain at least one H hydrogen for example. A concentrated acid is an acid which is in either pure form or has a high concentration. As it reacts with the hydrochloric acid it forms soluble calcium chloride.
Calcium is a metal thus calcium oxide is a metallic oxide which is basic in nature. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. The calcium carbonate is a white powder that mixes with water but does not dissolve.
Since calcium carbonate is relatively insoluble it tends to come out of solution. 01 g of calcium carbonate is added to excess hydrochloric acid. Decomposition of food is important in the human digestive system.
At 1200K calcium carbonate decomposes to give carbon dioxide and calcium oxide. It slightly dissolves in water. This can take place through both biological and.
Similarly when calcium carbonate reacts with hydrochloric acid increasing the concentration of the acid speeds up the rate of reaction as long as enough calcium carbonate is present. 240 cm 3 of carbon dioxide gas is collected. Acid Metal Oxide Salt Water.
Hydrochloric AcidACS ManufacturerSupplier Trade name. They dont cancel. When an acid such as hydrochloric acid reacts with calcium oxide neutralization reaction takes place and calcium chloride along with water is formed.
Decomposition of proteins lipids and carbohydrates into amino acids fatty acids and monosaccharides provides the body with the nutrients it needs to function. Decomposition is used to make other chemical compounds decomposition reaction of calcium carbonate CaCO with hydrochloric acid HCl. Limestone is a common type of carbonate sedimentary rock which is the main source of the material limeIt is composed mostly of the minerals calcite and aragonite which are different crystal forms of calcium carbonate CaCO 3Limestone forms when these minerals precipitate out of water containing dissolved calcium.
Hydrochloric acid is a monoprotic molecule with an acid-dissociation equilibrium constant K a three orders of magnitude greater than sulfuric acid indicating HCl is both strong and effective as an acid. Calcium carbonate also called limestone is an example of a metal carbonate used in the Solvay process. CaCO 3 CaO.
Beginarraylendarray CaCO 3 H 2 SO 4 CaSO 4 H 2 OCO 2. Hydrochloric AcidACS Created by Global Safety Management Inc. Laboratory type sulfuric acid about 98 by weight is a concentrated and strong acid.
BaSO4 is the least soluble. There are many exceptional kinds of limestone formed thru a ramification of tactics. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon dioxide.
It is insoluble in ethanol and acetic acid but soluble in dilute nitric acid and hydrochloric acid. Limestone is mostly made up of the mineral calcium carbonate CaCO3. S25358 Recommended uses of the product and uses restrictions on use.
H 2 SO 4 sulfuric acid. But if you add an acid you add hydrogen ions H which will react with the carbonate to form hydrogen carbonate HCO3- ions which are very soluble in water and the limestone will dissolve. The rate of this reaction depends on the amount of sulfur dioxide in the air.
It may be precipitated from water non-clastic chemical or inorganic limestone secreted by using marine organisms including algae and coral biochemical limestone or can shape from the shells of. When it reacts with dilute acid it liberates carbon dioxide as a by-product. Buy sulfuric acid for chemistry labs educational use drains batteries pools toilets plants and lawns Order Sulfuric Acid 96 ACS Reagent Grade Solution here.
Sulfuric acid barium hydroxide --- barium sulfate and water. You can also cause a double replacement chemical reaction when you combine an acid and a base. Or if there is more acid two hydrogen ions will.
Calcium bicarbonate entering with the feed water is broken down at boiler temperatures or reacts with caustic soda to form calcium carbonate. 3 Group II sulfates become less soluble down the group. You get immediate fizzing with a colourless gas given off - thats carbon dioxide.
When an acid such as hydrochloric acid reacts with calcium oxide neutralization reaction takes place and calcium chloride along with water is formed. The K a value for HCl is reported at 13 x 10 6 where sulfuric acid is 10 x 10 3. HCl hydrochloric acid.
The reaction stops after 15 seconds. Identification of the substancemixture and of the supplier Product name. For example calcium carbonate CaCO 3 deteriorates as a result of its reaction with the pollutant sulfur dioxide.
Calcium carbonate occurs naturally as chalk limestone and marble. Silver nitrate sodium chloride --- silver chloride and sodium nitrate. Through the ingestion of 05-15 grams in adults the magnesium hydroxide will act by simple acid neutralization in the stomach.
Reactions that use an acid and a base as reactants is known as a neutralization reaction. The hydroxide ions from the magnesium hydroxide suspension will combine with the acidic H ions of the hydrochloric acid made by the stomachs parietal cells. A hydrogen ion is just the proton and no electron.
Calcium is a metal thus calcium oxide is a metallic oxide which is basic in nature. Limestone is a sedimentary rock such as greater than 50 calcium carbonate calcite CaCO3. An acid is a substance that produces hydrogen H ions when it is added to water.
Similarly when sulphuric acid reacts with zinc oxide zinc sulphate and water are formed. Calcium carbonate is made up of 28 grams of calcium oxide and 22 grams of carbon dioxide. If acidified barium chloride is added to a solution that contains sulfate ions a white precipitate of barium sulfate forms.
Zinc reacts with dilute hydrochloric acid to produce zinc chloride solution and hydrogen gas which. It is found in. The photo shows the reaction with marble chips.
The commonest carbonate-acid reaction you will come across is that between calcium carbonate and dilute hydrochloric acid.

Write A Balanced Chemical Equation For The Reaction Of Calcium Carbonate And Dil Hydrochloric Acid

Question Video Calculating The Average Rate Of Reaction Of Hydrochloric Acid With Calcium Carbonate Nagwa

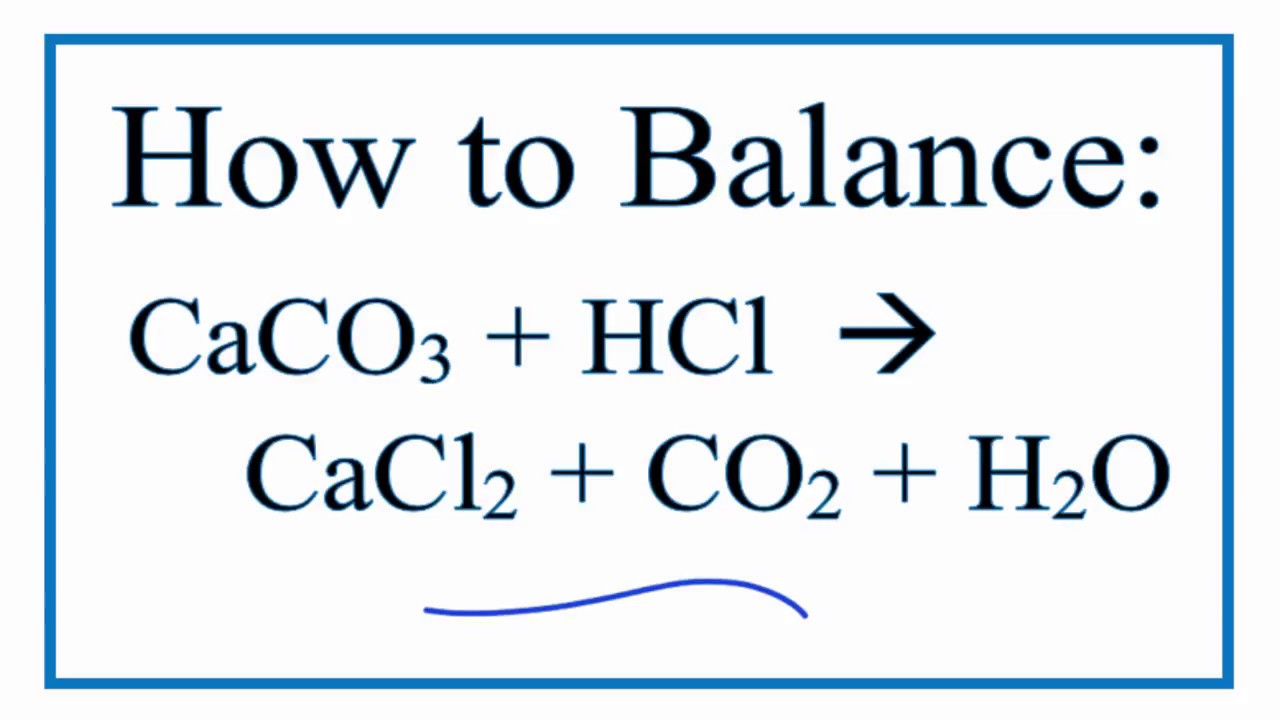
How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube
0 Comments